IHS Provides Updates on Timeline and Resources for Seasonal Influenza, RSV, and COVID-19 Vaccines
On September 22, 2023, the Indian Health Service (IHS) sent a Dear Tribal Leader Letter (DTLL) and Dear Urban Indian Organization Leader Letter (DULL) to provide an update concerning seasonal vaccines and resources available for vaccine-related activities to purchase COVID-19 vaccines. In the letter, IHS discussed vaccines for Influenza, Respiratory Syncytial Virus (RSV), and COVID-19.
IHS provided the following updates:
Influenza Vaccine
Supply of the 2023-2024 seasonal influenza vaccine is expected to be adequate to provide access for all recommended age groups, including the influenza vaccines preferentially recommended for people ages 65 years and older. The Centers for Disease Control and Prevention (CDC) continues to recommend the seasonal influenza vaccine for all people ages six months and older. September and October are the best times for most people to get vaccinated.
RSV Vaccine
Vaccination to prevent RSV is also available for certain high-risk persons. This includes approved RSV vaccines available for persons ages 60 years and older, and one of the RSV vaccines, ABRYSVO, is approved for use in pregnant individuals 32-36 weeks gestational age to prevent lower respiratory tract disease (LRTD) and severe LRTD in infants from birth through 6 months of age. There is also the long-acting monoclonal antibody, nirsevimab, which has been approved and recommended to prevent RSV for all infants under 8 months entering their first RSV season, and all American Indian and Alaska Native children ages 8-19 months entering their second RSV season.
COVID-19 Vaccine
In September, the Food and Drug Administration approved and authorized, and the CDC recommended, an updated monovalent mRNA COVID-19 vaccine designed to protect against the currently circulating strains of the virus. All people ages 6 months and older, regardless of prior COVID-19 vaccination status, are recommended to receive this vaccine. The vaccine is now available, so please do not wait to get your updated COVID-19 vaccine.
Like other adult vaccines, after regulatory approval/authorization and recommendation, the updated 2023-2024 COVID-19 vaccines (Pfizer, Moderna, and Novavax, once authorized) will be commercially available through the channels used to procure other routine vaccines. Updated COVID-19 vaccines are available in retail pharmacies.
Vaccines will be available to uninsured or underinsured adults through the HHS Bridge Access Program for a limited time. CDC’s Bridge Access Program provides no-cost COVID-19 vaccines to adults without health insurance and adults whose insurance does not cover all COVID-19 vaccine costs. This program will end by December 31, 2024.
Pediatric COVID-19 vaccines will continue to be available to all American Indian and Alaska Native children via the CDC’s Vaccines for Children (VFC) program. The VFC program is a federally funded program that provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay. The CDC buys vaccines at a discounted rate for distribution to registered VFC providers. Children who are eligible for VFC vaccines are entitled to receive those vaccines recommended by the Advisory Committee on Immunization Practices. Eligible children are children through 18 years of age who meet at least one of the following criteria are eligible to receive VFC vaccine:
- Medicaid eligible: A child who is eligible for the Medicaid program. (For the purposes of the VFC program, the terms “Medicaid-eligible” and “Medicaid-enrolled” are equivalent and refer to children who have health insurance covered by a state Medicaid program)
- Uninsured: A child who has no health insurance coverage
- American Indian or Alaska Native: As defined by the Indian Health Care Improvement Act (25 U.S.C. 1603)
- Underinsured
For any questions related to vaccines, please contact CAPT Kailee Fretland, Pharmacist, Office of Clinical and Preventive Services, IHS, by e-mail at kailee.fretland@ihs.gov.
For any questions pertaining to COVID-19 supplemental funding, please contact Ms. Jillian Curtis, Chief Financial Officer, IHS, by e-mail at jillian.curtis@ihs.gov.
NCUIH Vaccine Advocacy
The National Council of Urban Indian Health (NCUIH) has long supported equitable vaccination access for urban American Indian and Alaska Native people. With support from the CDC, NCUIH has been working to promote equitable adult vaccination and prevent severe illnesses such as COVID-19 and influenza. We do this by enhancing the resource and evidence base, developing effective strategies for health care organizations, and creating culturally appropriate materials for individual clinicians that reflect the needs of urban American Indian and Alaska Native people.
Join NCUIH on November 9, 2023, for the second session in our vaccine Community of Learning (CoL) series, “Paths to Vaccine Equity: Annual Vaccinations.” Speakers from NCUIH and Amy Pisani, CEO of Vaccinate Your Family, will discuss the new RSV vaccine and updates to the COVID-19 and influenza vaccines. Participants will learn how collaboration with vaccine organizations can help support vaccine awareness and patient education.
For more information on NCUIH’s vaccine advocacy work, please click here.


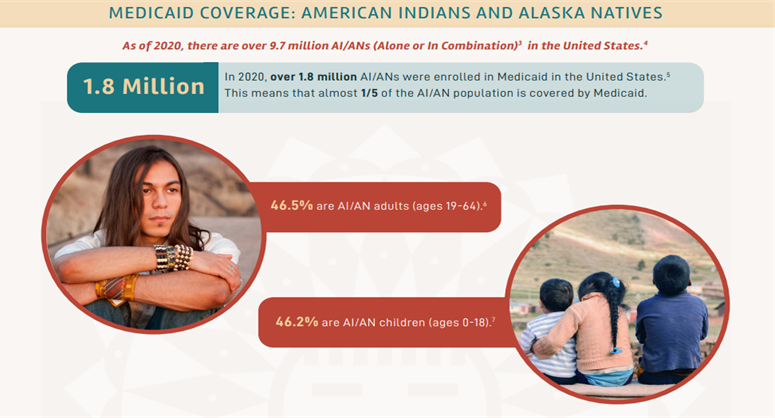
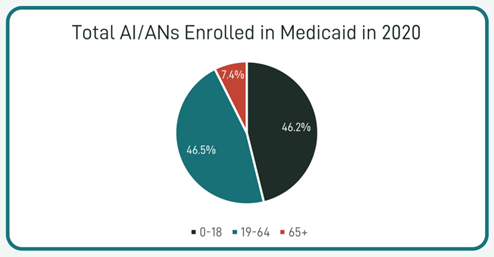
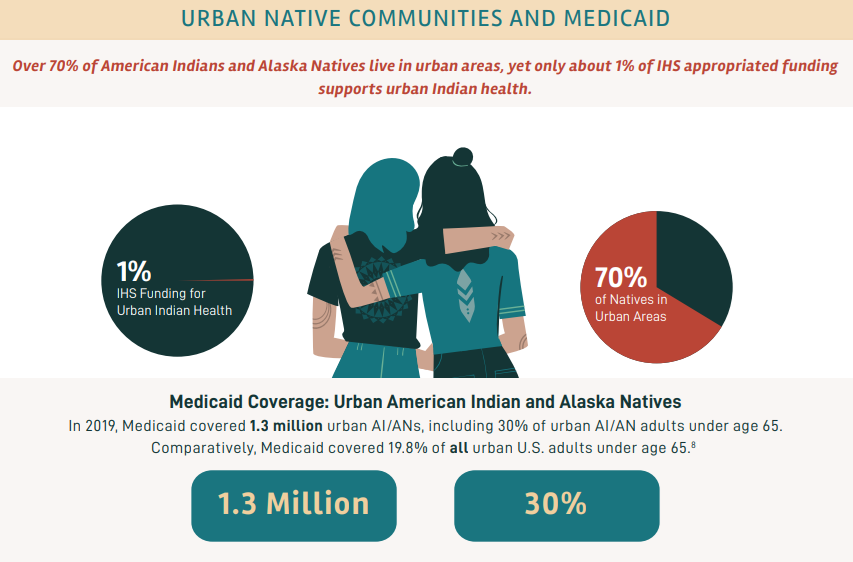
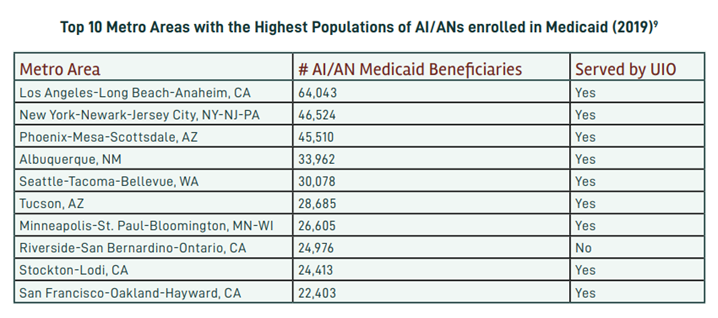

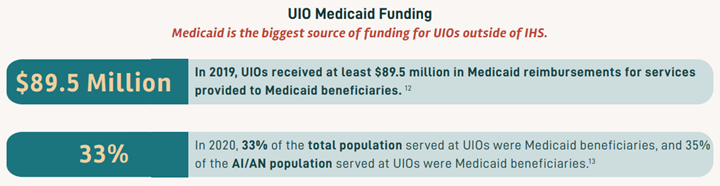
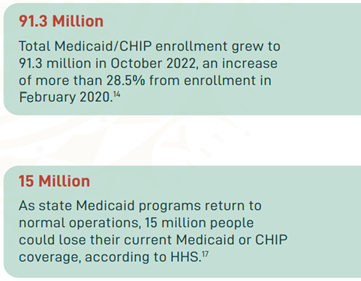 In March 2020, the Families First Coronavirus Response Act (FFCRA) Medicaid and Children’s Health Insurance Program (CHIP) “continuous coverage” requirement allowed people to retain Medicaid coverage and receive needed care during the COVID-19 Pandemic Public Health Emergency (PHE).
In March 2020, the Families First Coronavirus Response Act (FFCRA) Medicaid and Children’s Health Insurance Program (CHIP) “continuous coverage” requirement allowed people to retain Medicaid coverage and receive needed care during the COVID-19 Pandemic Public Health Emergency (PHE).
 The National Council of Urban Indian Health (NCUIH) is pleased to announce the release of its
The National Council of Urban Indian Health (NCUIH) is pleased to announce the release of its 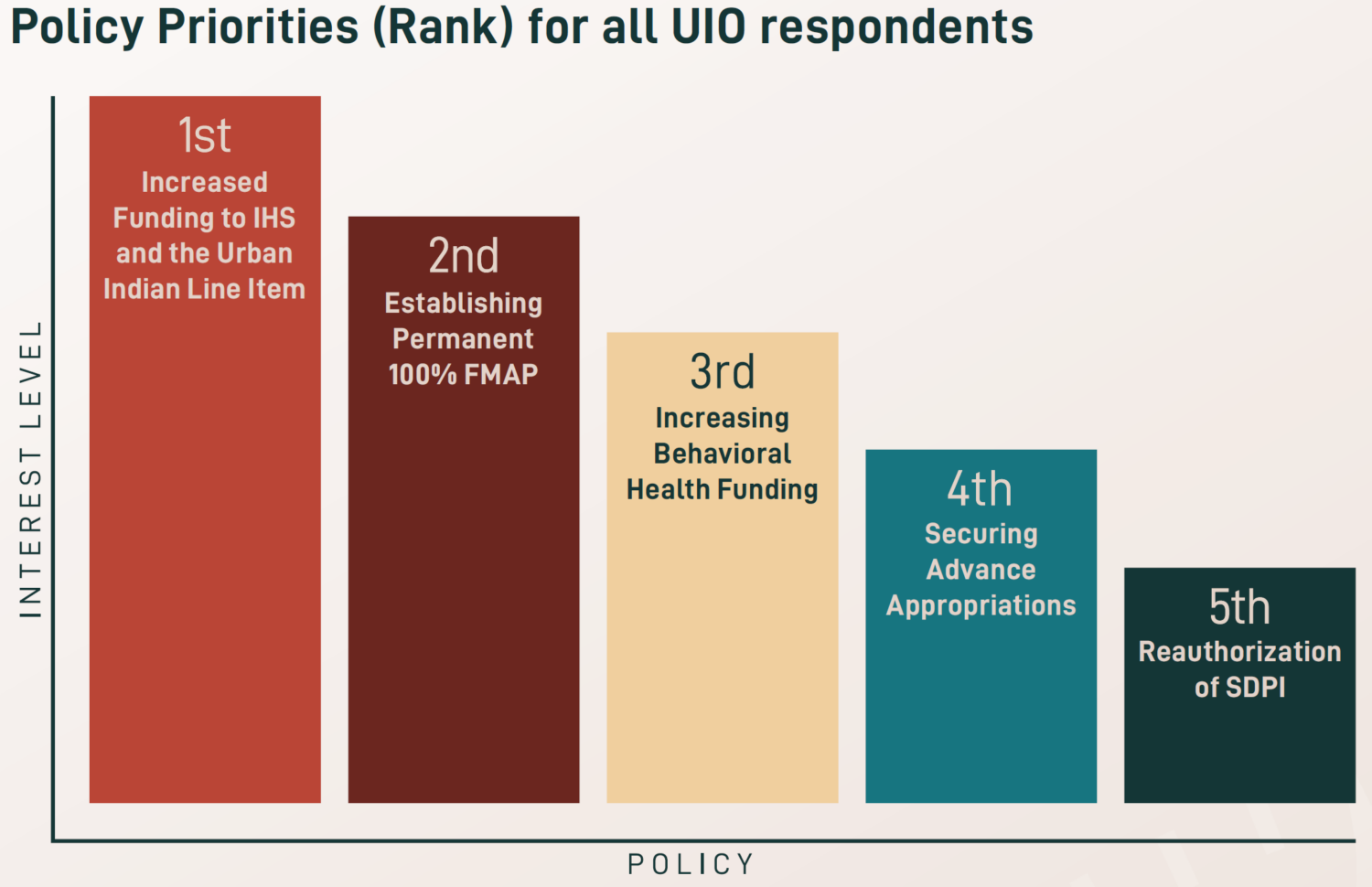 After the height of the COVID-19 pandemic, newfound priorities were identified for 2023, including workforce development and retention, increased funding for traditional healing, and expanded access to care and telehealth services. Existing priorities also remain a key focus across UIOs, especially increasing funding amounts for the urban Indian health line item and IHS, maintaining advance appropriations for IHS, establishing permanent 100% Federal Medical Assistance Percentage (FMAP) for UIOs, reauthorizing the Special Diabetes Program for Indians (SDPI), and increasing behavioral health funding.
After the height of the COVID-19 pandemic, newfound priorities were identified for 2023, including workforce development and retention, increased funding for traditional healing, and expanded access to care and telehealth services. Existing priorities also remain a key focus across UIOs, especially increasing funding amounts for the urban Indian health line item and IHS, maintaining advance appropriations for IHS, establishing permanent 100% Federal Medical Assistance Percentage (FMAP) for UIOs, reauthorizing the Special Diabetes Program for Indians (SDPI), and increasing behavioral health funding.


