Be a Good Relative: What We Learned
Wonderful news; the National Council of Urban Indian Health has just completed its Be a Good Relative Campaign (BAGR). NCUIH is committed to the continuation and promotion of vaccine equity for Urban Indians. The BAGR campaign was launched to provide educational material on vaccines culturally tailored to Native and Urban Native communities. This series of four videos, promoted on February 16th, April 20th, April 29th, and June 16th.
These videos have been one of our most successful campaigns yet, reaching many people thanks to enhanced promotion for the third and fourth videos. The first video (#BeAGoodRelative Campaign: Flu Immunization) had 311 total views, with 5 reshares and 23 likes. It received 1,318 impressions. The second video posted on April 20th (#BeAGoodRelative Campaign: COVID-19 Myths vs Facts) was viewed 4,473 times, retweeted 104 times, and liked 656 times. The link was clicked 75 times. The impressions on this video were 17,936 and the engagements were 1,275. The third video (#BeAGoodRelative Campaign: Annual Vaccines) had 249 total views, with 5 reshares and 4 likes. The last video (#BeAGoodRelative Campaign: Youth Immunization), was also quite successful. The video was viewed 3,182 times, retweeted 96 times, and received 472 likes. The link was clicked 53 times. The impressions on this video were 11,063 and the engagements were 819.
Feedback surveys showed that the second and final BAGR videos were effective at reaching and engaging the Urban Native community on vaccination.
One Third of respondents did not receive a COVID-19 vaccine. 66% were very likely to get vaccinated after watching the video, and only 5% were still not likely to get vaccinated.
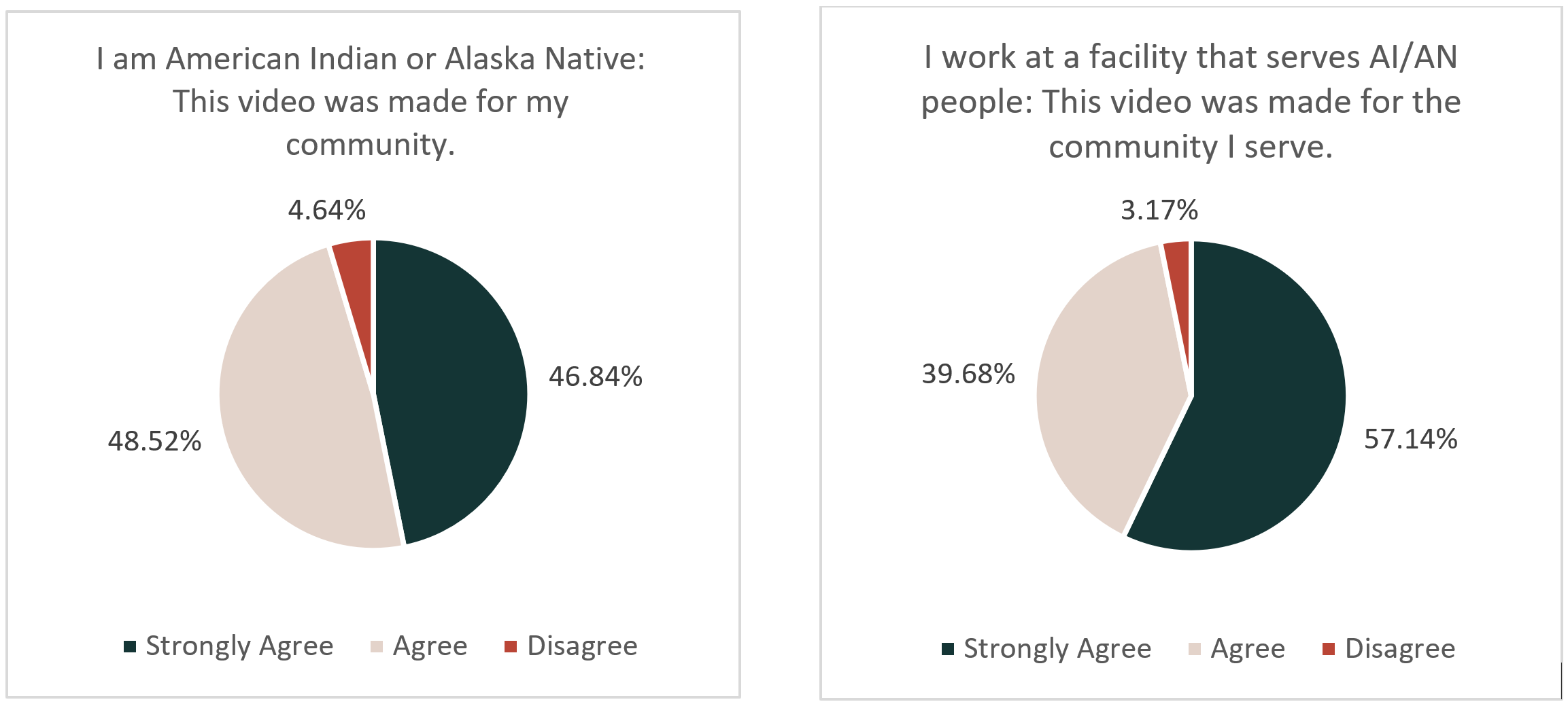
Further, of the 271 AI/AN people who responded to the feedback survey, they overwhelmingly agreed that the video represented their community. People who worked at facilities that serviced American Indians and Alaska Natives also agreed that these videos were representative of the communities they serve.
Respondents were also asked what factors were the most important when considering getting a vaccine. There was a range of sentiments, the most prevalent of which was a desire to protect oneself against the virus and the disease. However, there were also a substantial group of respondents who indicated concerns for the safety of the vaccine as well as potential side effects. Additionally, respondents indicated that they would consider the effectiveness of the vaccine in protecting against the virus, a desire to protect their family or community, as well as trying to stop the pandemic. Some of the less common concerns were the perceived cost of acquiring the vaccine, access to the vaccine and equity, as well as more trials for effectiveness and safety. Knowing this we can see what sort of messaging would be most receptive to our community.
NCUIH thanks the Urban Indian community and everyone who viewed and provided feedback to our #BeAGoodRelative Campaign. We have learned much about effective vaccination messaging for our community. Moving forward, we will continue to share materials to promote vaccination and vaccine equity that our community would find helpful and useful.

